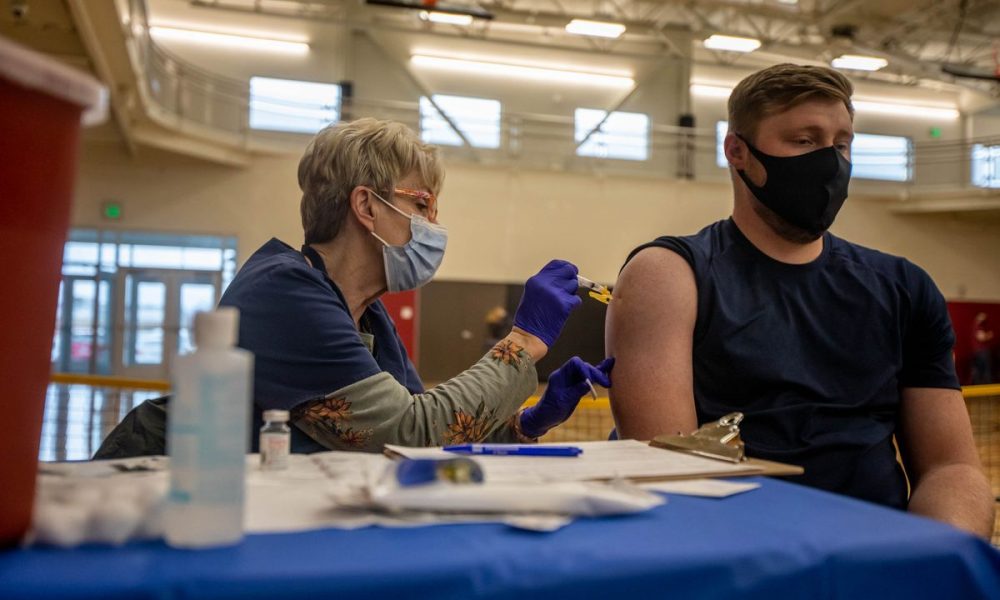
The company announced Monday its vaccine effectively prevents severe illness and hospitalizations across all age groups and ethnicities.
What We Know:
- The vaccine, developed in conjunction with Oxford University, underwent trials in the U.S., Peru, and Chile. The study included 32,449 individuals with just 141 developing symptomatic covid cases. It was administered at a 2:1 rate of vaccine to placebo. A news release from the company stated that vaccine efficacy was consistent across all age groups, with it specifically being 80% for those 65 and older.
- The news comes at a time when the company has experienced significant blowback to its vaccine; last week, several countries paused administering it after reports of blood clots surfaced. However, after reviewing the data, the European Medicines Agency announced that the vaccine was, in fact, safe, stating the benefits outweigh any possible risks.
- AstraZeneca may be unable to recover from the damage to its reputation. A poll conducted in Europe revealed that more people in Germany, Spain, France, and Italy now consider the vaccine to be unsafe. Only citizens in the United Kingdom, where the vaccine has been approved since December, have not been affected by the recent reports. 77% of people consider it to be safe, as reported by NBC.
- While the U.S. trials have shown the vaccine to be safe and effective, the study’s main benefit will be felt overseas. Over 50 countries had approved AstraZeneca’s use, and it is the core of a United Nations project called COVAX that seeks to bring covid vaccines to poorer countries. Currently, the U.S. has only approved vaccines from Pfizer, Moderna, and Johnson & Johnson.
- The company will now seek authorization from the Food and Drug Administration for emergency use. Because the vaccine has not been approved, 7 million of its doses have remained in storage. This led to the Biden administration announcing last week that millions of its extra doses will be shared with Canada and Mexico.
The U.S. signed a deal that, once approved, will provide the country with 300 million doses of the AstraZeneca vaccine as the country seeks to have doses for all adults by May.